What is fluoride?
Fluorides naturally arise from a compound containing fluorine gas. The element with which fluorine binds determines whether sodium, amine or stannous fluoride is formed. The compounds exhibit completely different properties than fluorine. In fact, fluorides are salts with positive properties, while fluorine is a toxic gas.
Where is fluoride found?
Fluoride is a naturally occurring substance, not an artificial compound. Fluorine salts are present not only in rock layers but also occur naturally in the human body as essential components of our bones and teeth.
Drinking water
Both mineral and tap water contain fluoride. How much fluoride depends on where you live. In some countries, tap water is fluoridated as an extra measure to safeguard the population against dental decay. However, this is not the case in most European countries. Tap water in Germany, for example, is considered low in fluoride, with a value of less than 0.3 mg per litre. Fluoride levels in mineral water differ significantly depending on the water's origin. Interesting fact: Seawater also contains fluoride – typically at an average concentration of 1 mg per litre.
Good to know:
Water with a fluoride content of up to 0.7 mg per litre is deemed appropriate for preparing baby food.
Digression: How is fluoride identified in drinking water?
The German Federal Institute for Materials Research and Testing (BAM) has developed a test strip for quickly determining the fluoride concentration in water. This test strip works similar to a pH test strip, indicating the fluoride concentration through a colour change.
In most European countries, however, this type of test is not necessary. Fluoride detection is more useful in countries where water is fluoridated but lacks consistent monitoring – for example, in certain regions of Asia and Africa. If you want to know how much fluoride is in your tap water, simply get in touch with your local water authority; there is no need to test the water yourself.
Fluoride in food
Trace amounts of fluoride are also found in food. Presented below is a list of food products with the highest fluoride content – based on the Federal Food Key of the the Max Rubner-Institut (MRI) (data in mg per 100 g):
- Fluoridated salt (47)
- White tea, dry (9.5)
- Mate tea, dry (9.5)
- Black tea, instant or dry (9.5)
- Green tea, dry (9.5)
- Peppermint tea and herbal tea, dry (7)
- Spirulina, powder or dried (0.92)
- Celery or sorrel, powder or dried (0.76)
- Seaweed powder (up to 0.69)
- Salmon (up to 0.69)
- Walnuts (up to 0.68)
- Sardines (up to 0.66)
- Brook trout (up to 0.648)
Guide values for fluoride
How much fluoride is healthy? The German Nutrition Society (DGE) has established recommended guide values for total fluoride consumption:
- Women: 3.1 mg per day
- Men: 3.8 mg per day
However, our typical dietary intake is notably lower: around 0.4 to 0.6 mg per day.
Fluoride in toothpaste and dental care products
Since 1850, the protective effects of fluoride-containing tooth enamel against acids have been recognised. Consequently, scientists realised that fluoride supplementation would help prevent dental decay. Fluoride has, therefore, been recommended for protection against dental decay since 1874.
However, research did not stop there. Meanwhile, over 300,000 scientific studies have explored fluoride's role in combating dental decay, resulting in an abundance of dental care products like gels, varnishes, mouthwashes and, of course, toothpaste containing sodium fluoride.
Good to know:
Did you know that thoroughly rinsing your mouth with water after brushing your teeth diminishes the effectiveness of fluoride in toothpaste? It is better to just spit out the toothpaste and to not rinse your mouth with water. This allows the fluoride to develop its full effect and optimally protect your teeth.
Effect: Fluoride in toothpaste - a key defence mechanism against dental decay
How exactly does fluoride protect the teeth against dental decay? First, we need to understand how dental decay initially develops.
Digression: How does dental decay develop?
The structure of tooth enamel
Despite being the hardest material in the human body, tooth enamel is remarkably sensitive. It consists of a super-fine crystalline lattice structure of a mineral known as hydroxyapatite. Minerals such as magnesium, sodium, calcium and phosphorus are bound within this crystalline lattice structure.
The mouth as a habitat
Millions of bacteria live in the oral cavity. The majority of them are "good" and essential for digestion. However, there are also "bad" cavity-causing bacteria that attack the tooth enamel. And this is how it works: These bacteria thrive on sugar, which they digest and excrete as acid. Thus, the pH value in the mouth changes and becomes acidic.
Good to know:
Cavity-causing bacteria thrive not only on sweets, cakes and chocolate. They also love fructose and lactose, which are contained in healthy foods such as fruit and dairy products.
Demineralisation and remineralisation
Upon acid secretion by bacteria, immediate neutralisation occurs, as minerals like calcium and phosphorus are released directly from the crystalline lattice structure of the tooth enamel. This process is referred to as demineralisation.
The problem: The loosening of the minerals creates gaps in the crystalline lattice structure of the tooth enamel that need to be filled. Saliva is responsible for this task. As soon as the bacteria are done with their acid attack, since the sugar has been digested and the oral pH value neutralised, the saliva is able to re-close the open gaps in the tooth enamel. Because besides water, saliva contains essential minerals such as calcium phosphates. The crystalline lattice structure of the tooth enamel is replenished and the actual tooth enamel becomes hard and durable again. This process of replenishing mineral levels is also called remineralisation.
The start of dental decay
Serious problems only start to occur when demineralisation and remineralisation are unbalanced. For instance, excessive sugar intake, persistent acid secretion by bacteria and an unbalanced oral pH value may overwhelm your saliva's capacity to replenish minerals. Bacteria can then settle in the crystalline lattice structure of the tooth enamel and easily multiply and spread. This is how dental decay develops.
How does fluoride protect against dental decay?
Fluoride protects tooth enamel in three different ways:
1. Fluoride as a remineralisation accelerator
With the help of fluorides, calcium phosphates can be absorbed more quickly in the tooth enamel after teeth demineralisation caused by an "acid attack". This helps to quickly close any weak spots in the crystalline lattice structure of the tooth enamel, giving bacteria less time to settle in the gaps. The actual fluorides are also stored in the tooth enamel. This means that the tooth enamel is well prepared for efficient and rapid remineralisation after the next acid attack.
2. Fluoride as a protective film against dental decay
Regular use of fluoride toothpaste forms a kind of protective coating over the teeth. Instead of neutralising the acidic pH value with minerals from the tooth enamel, the protective layer is initially attacked, not the actual tooth enamel. When brushing teeth, the fluoride is absorbed into the tooth enamel and replaces hydroxide ions. This results in the formation of a wafer-thin layer of a mineral called fluorapatite. In contrast to hydroxyapatite, which makes up a large percentage of the foundation of our tooth enamel, it is considerably stronger and more resilient. As such, fluoride protects the teeth against acid attacks. Interesting fact: Shark teeth are made mostly of fluorapatite and are, therefore, extremely strong.
3. Fluoride as a bacteria killer
Fluoride has an antibacterial effect, which helps to combat bacteria already on or in the tooth enamel. The fluoride penetrates the bacteria and manipulates their metabolism. Consequently, fewer bacteria are able to produce the acids that attack tooth enamel and, ultimately, cause dental decay.
Scientific consensus on the effectiveness of fluoride
The world of dental health is in agreement: Fluoride offers effective protection against dental decay. This is not only the view of the scientists behind the more than 300,000 studies published on the subject of fluoride and its impact on dental decay but also of official authorities and numerous consumer protection organisations, such as Stiftung Warentest, Germany's leading consumer testing organisation, all of whom recommend using fluoride toothpaste.
In the world of dentistry, it is also an undeniable fact that the use of fluoride toothpaste for brushing teeth is a primary reason for the drastic reduction in dental decay in recent decades. A comprehensive oral health study conducted by the German Dental Association in 2016 came to the conclusion that there were twice as many caries-free teeth in 2014 as in 1997. Besides better preventive dental care, fluoride toothpaste was also stated as one of reasons for this improvement in oral health.
Side effects: Is fluoride unhealthy?
So, if fluorides are so effective against dental decay and are recommended by leading health institutions, why do doubts still remain? Terms such as "nerve poison" or "neurotoxin" can frequently be heard. What effect does fluoride have on the rest of the body? And what happens if you accidentally consume too much fluoride?
Social media posts, internet forums and alternative health blogs are full of content like: "The truth about fluoride". They tend to list the various harmful side effects and use scare tactics to frighten consumers. Let us take a closer look at the actual points of criticism.
Result of overdosing: Fluorosis
Fluoride has been proven to effectively reduce dental decay, but you should adhere to the "less is more" principle and never exceed the recommended amounts. The exact recommendations for fluoride content in children's and adult toothpaste can be found in the table below.
Children, in particular, should not exceed the prescribed daily fluoride dosage. If children consume too much fluoride, they can develop what is known as fluorosis – an overdose of fluoride that leads to white spots on their teeth. This simply causes an excessive amount of minerals to be stored in the tooth enamel.
An overdose may occur, for example, if fluoride tablets, fluoride toothpaste, fluoridated salt and fluoridated drinking water are consumed at the same time and over an extended period.
A mild form of dental fluorosis, which can also occur in Europe, is generally harmless and merely an aesthetic "problem". In some regions of Africa and India – where drinking water contains very high levels of fluoride – more extreme forms of fluorosis are not uncommon. Sometimes, the spots on people's teeth in these regions are even brownish in colour.
Good to know:
For a long time, scientists debated the question of whether it is better to provide fluoride through toothpastes or tablets. Today, we know that toothpaste is more effective due to its direct contact with the tooth enamel. Fluoride tablets are not necessary if your child uses a fluoride toothpaste. Important: Never give your child fluoride tablets and fluoride toothpaste at the same time. Simply choose which option is best for your child.
In case of extreme fluoride intake: Bone fluorosis
If people ingest a particularly large amount of fluoride over a longer period of time, it can lead to bone fluorosis, or skeletal fluorosis. In this case, bone density is abnormally elevated. However, this results in bones losing their natural elasticity, which can ultimately lead to restricted movement.
A report by the U.S. National Research Council indicated that crippling skeletal fluorosis might occur in people who have ingested 10 to 20 mg of fluoride per day for 10 to 20 years.
These values are very high and can only be achieved by drinking highly fluoridated water. Seeing as most European countries do not fluoridate their drinking water, you do not need to worry about skeletal fluorosis. This has also been confirmed by Stiftung Warentest, Germany's leading consumer testing organisation. According to the consumer protection organisation, products that help prevent dental decay cannot damage your bones.
Is fluoride toxic?
The dose makes the poison. The early modern physician Paracelsus came to this conclusion as early as the 16th century, and it remains as relevant to fluoride today as it did then. Yes, fluoride is poisonous, but only to a very limited extent.
For someone weighing 70 kg, experiencing the initial symptoms of fluoride poisoning, like nausea, abdominal pain, headache, diarrhoea, vomiting and drowsiness, would require them ingesting at least 350 mg of fluoride. The potentially toxic dose is 5 mg of fluoride per kilogram of body weight. In our case, that would be the equivalent of consuming two to three tubes of toothpaste.
A lethal dosage of fluoride would require the consumption of 33 to 67 tubes of toothpaste by the same individual, with them likely falling into a coma or suffering convulsions first.
But what about children? The German Federal Institute for Risk Assessment (BfR) determined that a child may have an upset tummy at worst after consuming a whole tube of children's toothpaste (with a fluoride concentration of 0.05 per cent). Poisoning by fluoride is therefore unlikely. Nevertheless, it is advisable to restrict your child's access to fluoride toothpaste to prevent any risks.
What we can say is that fluoride is poisonous when large amounts are ingested, yet entirely harmless when used properly. In fact, according to the German Dental Association, it is almost ten times less toxic than table salt.
Good to know:
Often, the misconception that fluoride is detrimental to health or toxic comes from it being confused with the genuinely toxic gas, fluorine. Fluoride, on the other hand, is an entirely different substance with completely different properties.
Fluoride during pregnancy: Harmful to the brain?
A 2017 scientific study by the U.S. National Health Institute, various universities and government health authorities raised concerns among expectant parents. The study investigated whether heightened fluoride intake during pregnancy has an impact on child IQ.
The result: Excessive fluoride intake can diminish a child's long-term IQ. This study, however, did not refer to toothpaste but to drinking water in Mexico characterised by significant and unregulated fluctuations in fluoride content.
Expectant parents can therefore breathe a sigh of relief: The study subjects consumed significantly higher levels of fluoride than typical in most European countries. Brushing teeth with fluoride toothpaste during pregnancy is not linked to any reduction in child IQ.
Is fluoride harmful to the pineal gland?
Situated in the middle of the brain, the pineal gland, though tiny, plays a significant role: It produces the sleep hormone melatonin, and its main job is to help control important bodily functions, such as the circadian rhythm that regulates the sleep-wake cycle and the onset of puberty.
Fluoride critics claim that fluoride triggers pineal gland calcification. In fact, numerous studies have indeed discovered elevated fluoride concentrations within the pineal gland. Nonetheless, the causative role of fluorides in triggering calcification remains somewhat uncertain, as they might simply have adhered to pre-existing calcification – similar to tooth enamel remineralisation.
The important thing here, though, is: This criticism is founded on drinking fluoridated water, not using fluoride toothpaste. Nonetheless, the potential adverse impact of fluoridated water on the pineal gland and subsequent sleep problems cannot be dismissed. That said, the amount of fluoride the body absorbs through the use of toothpaste is extremely low, making it highly unlikely that your fluoride toothpaste will cause you any sleepless nights.
Is fluoride harmful to the thyroid gland?
In high doses, fluorides can indeed impede correct functioning of the thyroid gland. However, the fluoride content in commercially available toothpaste is so minimal that any concerns are completely unwarranted, particularly given that the majority of toothpaste on the brush is not swallowed. A potential link between fluoridated drinking water and hypothyroidism remains uncertain and is a topic of ongoing scientific debate.
Can fluoride cause a rash?
Perioral dermatitis is a red rash that appears around the mouth and on the chin, resembling acne. It primarily affects women aged 16 to 50 and children. The causes of this condition are unclear, but the suspected factors include using fluoride toothpaste, drinking fluoridated water, taking the contraceptive pill and applying oily facial creams. This fluoride intolerance is harmless but profoundly discomforting for those affected.
If you suffer from a rash around your mouth, avoid using a toothpaste with fluoride and oily facial products, at least temporarily, and seek medical advice. Your doctor will probably also prescribe an antibiotic cream or antibiotic tablets to treat the rash.
Zero fluoride toothpastes: Yes or no?
Zero fluoride toothpastes are popular at present. But what is fuelling the interest in them? Consumers are confused by rumours regarding fluoride's adverse effects on the body. Are zero fluoride toothpastes really better and healthier?
Disadvantages of toothpastes without fluoride
The disadvantage of zero fluoride toothpastes is quite obvious: The three protective effects of fluoride no longer apply. Zero fluoride toothpastes lack the ability to coat the tooth enamel with a protective layer, to accelerate remineralisation and to combat oral bacteria. Consequently, both Stiftung Warentest and Öko-Test, two leading German consumer testing organisations, consider zero fluoride toothpastes less effective in protecting against dental decay.
Advantages of toothpastes without fluoride
If you refrain from using a fluoride toothpaste, there is no risk of fluorosis – at least not through the toothpaste. The fact is: There is no "fluoride deficiency" in the human body as such. Fluoride is not essential for our survival. Many consumers opt for a zero fluoride toothpaste as part of a journey to embrace more natural products, devoid of the potentially detrimental additives commonly found in conventional fluoride toothpastes.
Good to know:
Even if you are a little worried about the harmful ingredients in your toothpaste, you do not have to miss out on fluoride's efficacy in preventing dental decay. Curaprox fluoride toothpastes are free from harmful substances such as SLS, microplastics and triclosan.
We definitely agree with the proponents of zero fluoride toothpaste on another aspect: Thorough mechanical cleaning with a toothbrush is much more important than the composition of your toothpaste. Good and thorough dental care is also possible using a zero fluoride toothpaste. Nonetheless, employing the correct toothbrushing technique and maintaining a regular dental care routine are crucial to preventing cavity-causing bacteria from colonising in your tooth enamel.
Who should use a zero fluoride toothpaste?
Zero fluoride toothpastes are particularly suitable for individuals who already consume an ample amount of fluoride through alternative sources, such as children taking fluoride tablets or people who live in areas with heavily fluoridated drinking water. Children taking fluoride tablets can also use a special zero fluoride children's toothpaste.
Good to know:
The zero fluoride toothpaste Enzycal zero from Curaprox contains three enzymes that are also found in saliva, offering a natural and gentle approach to cleaning. This helps to stimulate saliva production and thus also the remineralisation of tooth enamel. This toothpaste is also ideal for individuals suffering from very sensitive teeth, dry mouth, aphthae or irritation of the oral mucosa.
Four factors for selecting the right toothpaste
Have you ever stood in the toothpaste section of a supermarket or pharmacy and asked yourself the following question? Which product is the best one for me? The choice of toothpaste often comes from more of a gut feeling, or you end up buying the product with the most appealing packaging. The enormous choice is overwhelming. After all, the question about "toothpaste with or without fluoride" is not the only thing you need to consider when buying toothpaste. Discover the four factors you should pay attention to for picking the right toothpaste:
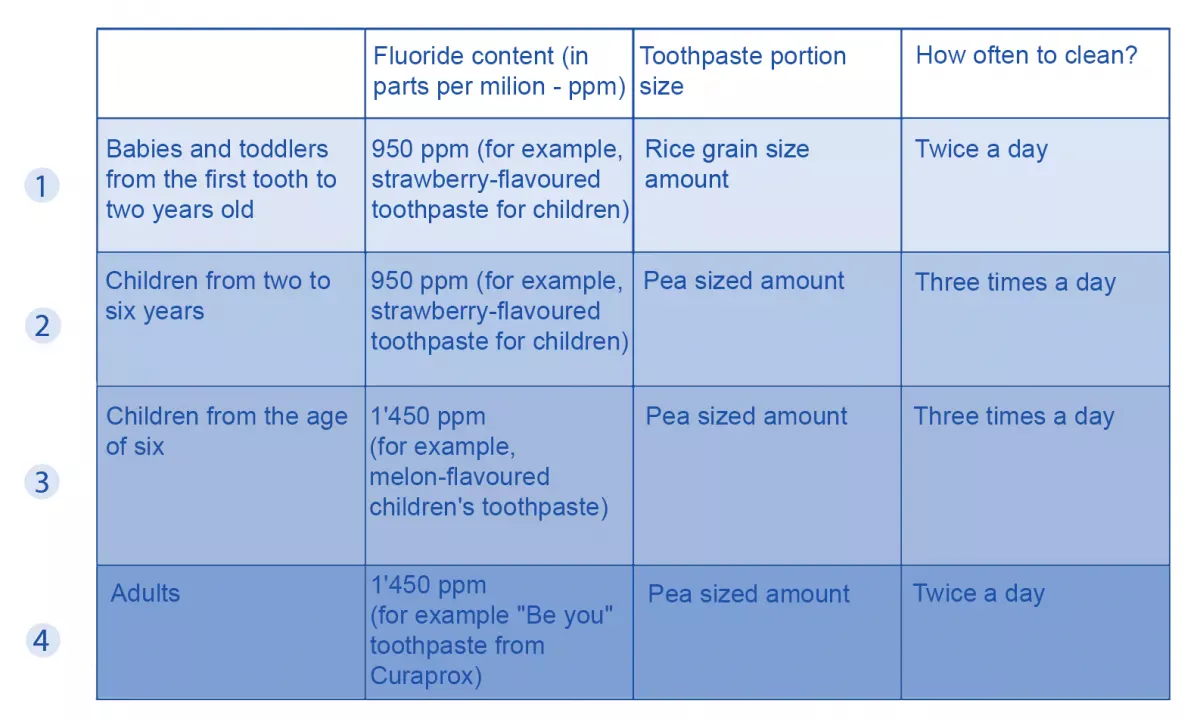
1. Recommended amount of fluoride in toothpastes for babies, children and adults
If you decide to buy a toothpaste with fluoride, the next question is: How much fluoride should it contain? If you have children, even more questions arise: When should you start brushing their teeth with fluoride? How much fluoride may the toothpaste contain? And how much toothpaste should you actually use for toddlers and babies? To answer these frequently asked questions as clearly as possible, we have prepared a table showing the recommended fluoride content in each case.
Based on the revised guidelines of the European Academy of Paediatric Dentistry (EAPD), we recommend the following fluoride content when brushing teeth:
Caution: The values for children only apply if you are not giving your child fluoride tablets. If you are giving your child fluoride tablets and fluoride toothpaste at the same time, there is a risk of fluorosis.
2. RDA value
The RDA value (relative dentin abrasivity) indicates the extent to which a toothpaste can wear away your tooth enamel. Some toothpastes contain abrasives that can damage the enamel – similar to a skin peeling treatment, but with the big difference that your tooth enamel will not grow back.
Evaluate the RDA values as follows:
- Below 70: Low abrasive effect; also suitable for sensitive teeth.
- 70 to 100: Normal abrasive effect; suitable for daily use with healthy teeth.
- Above 100: High abrasive effect; not suitable for daily use.
Ideally, the RDA value should be between 40 and 80. Toothpastes with a whitening effect usually have a much higher RDA value and thus a higher abrasive effect. In this category, values of up to 100 are permitted. People with particularly sensitive teeth should use a toothpaste with an abrasive value of less than 50.
Good to know:
Besides tasting exceptionally good, the Curaprox 'Be you' toothpastes whiten teeth naturally. Their RDA value of 50 is particularly low for a whitening toothpaste, and they do not harm the tooth enamel.
3. Ingredients
Many people are worried about fluoride in toothpaste. However, it is not the ingredient you should be concerned with the most:
- SLS (sodium lauryl sulphate): This active ingredient is responsible for the strong foaming effect of conventional toothpastes. Unfortunately, this gives us a misleading sense of cleanliness. Further, SLS attacks the oral mucosa and upsets the intestinal flora.
- Microplastics: The plastic particles, which are less than five millimetres in diameter, are a threat to both the environment and living organisms – despite this fact, they are used as abrasive microplastic beads in certain brands of toothpaste. Due to environmental pollution with plastic waste, microplastics have already been found in animals and drinking water. When it comes to cosmetics and care products, you should always opt for plastic-free ones whenever possible.
- Triclosan: Triclosan has an antibacterial effect and also helps to combat fungi. This active ingredient is often found in conventional toothpastes to help prevent gum inflammation. However, it makes the bacteria more resistant to our natural defences and is suspected of promoting cancer growth.
- Bleaching agents: Large quantities of aggressive bleaching agents can quickly have a whitening effect. However, they also attack the tooth enamel and result in a chemical imbalance, thereby making your teeth easy prey for cavity-causing bacteria.
- Parabens: Parabens are used to boost the shelf life of toothpaste. They help protect against microorganisms. However, they interfere with the body's hormones and are suspected of promoting breast cancer.
When choosing a toothpaste, make sure it does not contain any of the aforesaid substances.
Digression: Is titanium dioxide carcinogenic?
Due to genotoxicity concerns, titanium dioxide has been banned in food and food supplements in the EU since August 2022. It was previously used as food additive E 171 in chewing gum, coated tablets and baked goods.
The whitening and brightening agent fell into disrepute after studies with rats showed that the agent could be carcinogenic if high concentrations of titanium dioxide are inhaled. At one time, products containing titanium dioxide in powder form had to display the warning label "possibly carcinogenic to humans". However, the European Court of Justice (ECJ) has since declared this regulation null and void.
For instance, titanium dioxide may still be used in cosmetic products, such as sunscreens. Titanium dioxide is also used as a whitener in toothpastes. And there is a risk of toothpaste being swallowed, especially by children. Therefore, the Curaprox kids toothpastes do not contain titanium dioxide.
4. Flavour and sensation of freshness
Even if you are not supposed to swallow toothpaste, its flavour is an important criteria. After all, brushing your teeth should be fun, not a chore. Toothpaste must taste delicious and leave a pleasant and long-lasting refreshing taste in your mouth after brushing. If this is not the case with your current toothpaste, try out a new one. It will make adhering to your daily toothbrushing routine much easier.
Good to know:
Fancy trying a gin tonic or apple flavoured toothpaste? The Curaprox 'Be you' toothpastes come in six different flavours. The 'Be you' Six Taste Pack from Curaprox in a convenient travel size allows you to sample the entire range and discover your favourite toothpaste.
Summary: Are toothpastes with fluoride harmful?
No, toothpastes with fluoride are not harmful – provided you stick to the recommended values and do not eat whole tubes of them. It is an undeniable fact that fluoride serves to prevent dental decay and is a primary reason for the drastic reduction in dental decay in recent decades. Dentists, consumer protection organisations and public authorities all agree on this: The use of toothpaste with fluoride is explicitly recommended.
Most of the criticism towards fluoride relates to fluoridated drinking water and not to brushing teeth with a fluoride toothpaste. Since drinking water is not usually fluoridated in Europe and tap water is low in fluoride, there is no risk of overdosing as long as you do not give your child fluoride tablets and fluoride toothpaste at the same time.
That said, if you give your child fluoride tablets and want to continue doing so, or if you live in an area with fluoridated drinking water, it is probably a good idea to switch to a zero fluoride toothpaste. Otherwise, a fluoride toothpaste is the right choice to protect your teeth against dental decay. We recommend using a fluoride toothpaste with natural ingredients.
FAQs
Toothpaste with or without fluoride? A controversial topic. So, it is perhaps not surprising that it throws up so many questions. Below we have provided some frequently asked questions and answers:
Who invented toothpaste without fluoride?
Actually, zero fluoride toothpaste has been around longer than fluoride toothpaste. The first toothpaste, resembling what we use today, was invented by Washington W. Sheffield, an American dental surgeon, in 1850. He was the first person to enrich the toothpaste powder, which was commonly used at the time, with glycerine, thus creating a paste-like consistency. Initially, his revolutionary invention was available in metal and ceramic tins and tin foil bags. However, the toothpaste dried out very quickly. While studying dental surgery in Paris, his son, Lucius, realised that the collapsible tubes used by local artists to squeeze paint onto palettes would be ideal for his father's toothpaste. Hence, the first tube of toothpaste was born.
Will fluoride toothpaste be banned?
No, quite the contrary: Government authorities, scientists and medical associations all agree that fluoride is an essential element in the fight against dental decay and even expressly recommend its use.
Is the fluoride in toothpaste carcinogenic?
No, there is no evidence that fluoride in toothpaste could be carcinogenic. According to Stiftung Warentest, Germany's leading consumer testing organisation, there is also no increased risk of cancer in countries with fluoridated drinking water.
Which zero fluoride toothpaste should I buy?
Seeing as zero fluoride toothpaste lacks the fluoride that serves to prevent dental decay, it is best to choose a toothpaste that protects your teeth with xylitol, hydroxyapatite or enzymes and that contains no harmful substances. To find your ideal product, you can, for example, refer to various tests, take a closer look at the toothpastes deemed the best and read what others have to say about them. Your pharmacist will also be able to provide advice.
Our recommendation: The toothpaste Enzycal Zero from Curaprox contains three enzymes also found in saliva that stimulate the flow of saliva. They, therefore, boost the antibacterial and remineralising effect of saliva and protect your tooth enamel in a natural fashion.
Sources
Bashash, Morteza et al.: Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6-12 Years of Age in Mexico, in Environmental Health Perspectives. 2017.
Birr, Cornelia: Hashimoto: Wenn die Schilddrüse sich selbst zerstört, at: mdr.de.
Federal Institute for Health Protection of Consumers and Veterinary Medicine (BgVV): Verwendung fluoridierter Lebensmittel und die Auswirkung von Fluorid auf die Gesundheit.
Federal Institute for Risk Assessment (BfR): Risiko Vergiftungsunfälle bei Kindern.
Federal Ministry of Food and Agriculture (BMEL): Bundeslebensmittelschlüssel.
German Dental Association: Fünfte Deutsche Mundgesundheitsstudie (DMS V) – Kurzfassung.
German Dental Association: Verwendung fluoridhaltiger Zahnpasta ist sicher und schützt wirksam vor Karies.
Chemie.de: Neuer Schnelltest für Fluorid-Nachweis im Trinkwasser.
The German Nutrition Society (DGE): Fluorid.
Freund, Alexander: Wie gefährlich ist Titandioxid?, at: dw.com
German National Association of Statutory Health Insurance Physicians (KZBV): Zahnschutz durch Fluoride.
Nährwertrechner.de: Suchergebnis (nach Fluorid absteigend).
Nguyen-Kim, Mai Thi: Komisch, alles chemisch! Handys, Kaffee, Emotionen – wie man mit Chemie wirklich alles erklären kann. Droemer Verlag, Munich 2019.
O’Mullane, D.M. et al.: Fluoride and Oral Health, in: Community Dental Health. 2016.
Quarks: Darum hilft Fluorid bei der Kariesvorsorge.
Raschke-Maas, Kathleen: Schutz oder Gift? Wieviel Fluorid brauchen wir?, at: mdr.de.
Rehberg, Carina: Fluorid - Spurenelement oder Gift?, at:zentrum-der-gesundheit.de.
Salz, Melanie: Fluoride: Wie Sie Mythen in der Beratung souverän begegnen, at: coliquio.de.
Sjögren, K. et al.: The influence of rinsing routines on fluoride retention after toothbrushing, in: Gerodontology. 2001.
Steinert, Jürgen et al.: Macht uns Fluorid in Zahnpasta krank?, at: oekotest.de.
Stiftung Warentest: Das braucht es für gesunde Zähne.
Stiftung Warentest: Sind Fluorid, Zink und Titandioxid gefährlich?.
Tagesschau: Titandioxid zu Unrecht als krebserregend eingestuft.
Toumba, K.J. et al.: Guidelines on the use of fuoride for caries prevention in children: an updated EAPD policy document, in: European Archives of Paediatric Dentistry. 2019.
Zm: Sieben Mythen über Fluorid auf den Zahn gefühlt.
All websites last accessed on 9 May 2023.
 Swiss premium oral care
Swiss premium oral care